Protected Health Information (PHI) includes identifiable health data, protected by laws like HIPAA. PHI cannot be freely shared for research without anonymization and consent.
In this article, we’ll explore Protected Health Information (PHI), its significance in healthcare, and identify which statements about PHI are false, helping you understand the importance of safeguarding patient data.
What Is Protected Health Information (PHI)?
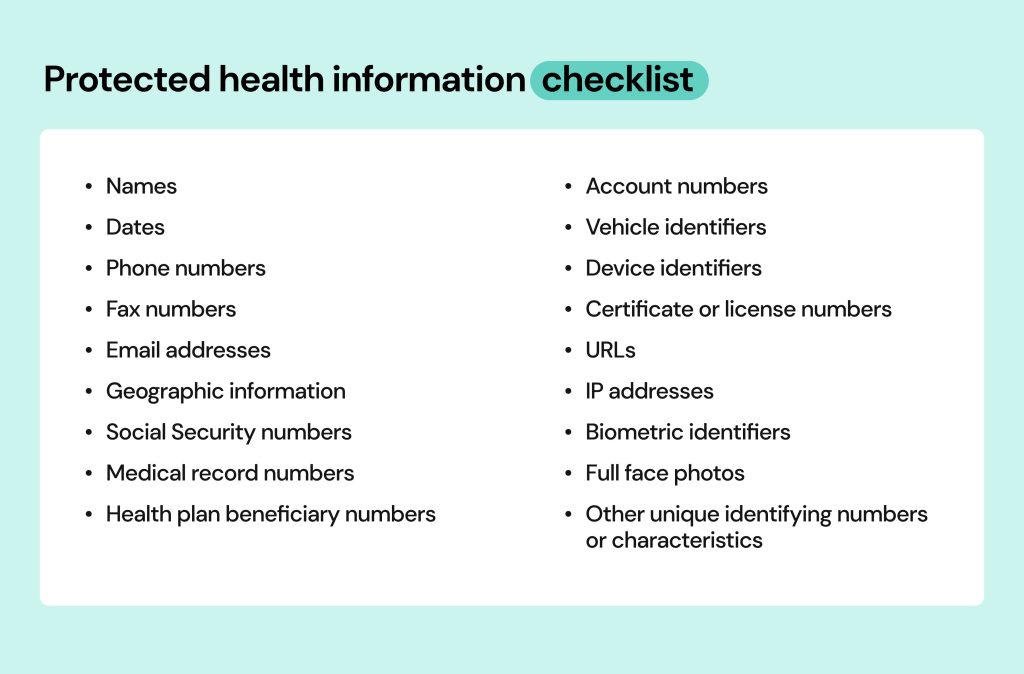
Protected Health Information (PHI) refers to any personal health data that can identify an individual and is related to their health, medical treatment, or payment for healthcare services. In the U.S., PHI is protected by the Health Insurance Portability and Accountability Act (HIPAA), which ensures privacy and security. PHI includes medical records, test results, billing information, and more, and can exist in various forms such as electronic, paper, or spoken information, all of which are safeguarded under privacy laws.
Key Examples of PHI:
- Personal Details: Name, address, phone number, email.
- Health Information: Diagnoses, treatment plans, prescriptions, lab results.
- Medical History: Previous surgeries, allergies, and any history of medical conditions.
- Insurance Information: Health insurance ID numbers, billing details.
The Importance of Protecting PHI:
Ensuring the confidentiality and security of PHI is essential for several reasons:
- Patient Privacy: Patients trust healthcare providers to keep their medical information private. When PHI is protected, patients feel more comfortable sharing sensitive information with healthcare professionals.
- Prevention of Fraud and Identity Theft: Unauthorized access to PHI can lead to identity theft or fraudulent activities. Protecting PHI reduces the risk of such incidents.
- Compliance with Laws: In many countries, healthcare organizations are legally required to safeguard PHI. In the United States, violations of the HIPAA law can result in severe penalties.
Identifying False Statements About PHI:

There are several common misconceptions about PHI, some of which could lead to confusion or unintentional violations of privacy rules. Below, we will explore some statements about PHI and identify which ones are false.
PHI includes only medical records stored in physical formats:
This statement is false. PHI can be in any form, including physical records and electronic files. It includes paper documents, like medical charts, and digital data, such as files on computers or shared over the internet. HIPAA’s Security Rule focuses on protecting electronic Protected Health Information (ePHI), which has specific security requirements to prevent unauthorized access, ensuring all forms of PHI are protected.
PHI can be shared freely if the patient provides verbal consent:
This statement is false. Verbal consent alone isn’t enough to share PHI. Written consent is usually required, except in specific cases like emergencies or public health reporting. PHI can only be shared for treatment, payment, or healthcare operations. If the data is de-identified (no identifying details), it can be shared freely. The Privacy Rule ensures consent is documented in writing to protect patient privacy.
PHI can be shared between healthcare providers without patient authorization if it is for treatment purposes:
This statement is true. PHI can be shared between healthcare providers without patient authorization if necessary for treatment. For example, a doctor may share a patient’s information with a specialist to provide appropriate care. This helps in coordinating treatment and ensuring the patient’s needs are met. However, any sharing beyond treatment requires patient consent or must follow legal guidelines, such as for research or marketing purposes.
Also read: Ma Health Connector – An Easy Guide To Health Coverage In Massachusetts!
It is acceptable to leave a voicemail with a patient’s medical information if they have provided their phone number:
This statement is false. Leaving medical information in a voicemail without explicit written consent is a breach of PHI privacy. While some patients may prefer phone contact, healthcare providers must avoid disclosing sensitive health details unless it’s done securely. To protect patient privacy, many use secure messaging systems, ensuring no unauthorized individuals overhear confidential health information, preventing accidental disclosures.
It is acceptable to share PHI with family members if the patient is deceased:
This statement is false. PHI remains protected under HIPAA even after a patient’s death. Healthcare providers cannot share PHI with family members without legal authority or the patient’s consent. PHI may be shared if the family member proves legal rights, like managing the estate or funeral arrangements, but it’s not automatic. Providers must follow HIPAA guidelines to ensure compliance when releasing information after death.
PHI can be shared for research purposes if the information is anonymized:
This statement is true. PHI can be used for research if it is anonymized or de-identified. This means all personal identifiers, like names or addresses, are removed, making it impossible to trace the data back to an individual. If data is anonymized, it is no longer protected under HIPAA. However, if the data isn’t anonymized, researchers must get explicit consent from patients to use their health information for research purposes.
Common Statements About PHI:
- PHI Includes Only Physical Medical Records: This statement is false. PHI encompasses physical, electronic, verbal information, and even data shared through emails or faxes. With digital records becoming more common, it is essential to protect all forms of PHI from unauthorized access.
- PHI Is Only Protected During the Treatment Phase: This statement is false. PHI is protected at every stage: during collection, storage, sharing, and even after treatment. Laws like HIPAA ensure that PHI remains confidential and secure throughout its lifecycle.
- Only Healthcare Providers Have Access to PHI: This statement is false. While healthcare providers do access PHI, other entities like insurance companies, billing agencies, and government bodies may also access PHI under specific conditions. These entities must also follow strict privacy rules to protect PHI.
- PHI Can Be Shared Without Consent in Emergency Situations: This statement is true but needs clarification. In emergencies, healthcare providers can access and share PHI without consent if the patient is unconscious or unable to consent. However, PHI shared in emergencies is limited to what is necessary for immediate care.
- Patients Have the Right to Access Their Own PHI: This statement is true. Patients can request copies of their medical records, request corrections, and even have their information transferred to other providers. Some restrictions may apply if sharing could harm the patient or others.
- PHI Can Be Shared Without Limitations for Research Purposes: This statement is false. PHI can only be shared for research purposes with strict guidelines and patient consent. Data must often be de-identified to ensure privacy, and patients must approve its use for research purposes to maintain confidentiality.
Which of the following statements is not true regarding protecting patient health information?
The statement “PHI can be shared without limitations for research purposes” is not true. While Protected Health Information (PHI) can be used for research, it is subject to strict guidelines. Researchers must typically obtain patient consent before using PHI for research, and the data should be de-identified to protect patient privacy. If the data is not anonymized, it remains protected under HIPAA regulations. Therefore, PHI cannot be shared without limitations, especially when it involves research, unless these safeguards are in place.
FAQ’S
What is Protected Health Information (PHI)?
PHI refers to any personal health information that identifies an individual, including data about their health, treatment, or healthcare payment.
Can PHI be shared without patient consent for treatment?
Yes, PHI can be shared between healthcare providers without patient consent if necessary for treatment or coordinated care purposes, ensuring patient safety.
Is it acceptable to leave medical information in a voicemail?
No, leaving medical information in a voicemail without written consent violates PHI privacy rules, as it risks unauthorized access to sensitive health details.
Can PHI be shared after a patient’s death?
No, PHI remains protected under HIPAA even after death, and can only be shared with authorized individuals based on legal authority or patient consent.
Can PHI be shared for research purposes?
PHI can be shared for research if anonymized or de-identified. Explicit patient consent is necessary if the data is not de-identified for research purposes.
Conclusion
In conclusion, Protected Health Information (PHI) must be carefully protected to ensure patient privacy and compliance with HIPAA laws. False statements, such as PHI being freely shared for research without consent, emphasize the importance of understanding privacy rules. It’s crucial to follow proper consent protocols when sharing PHI, whether for treatment, research, or communication, to maintain security and confidentiality.




